pH
WHAT IS IT?
About pH
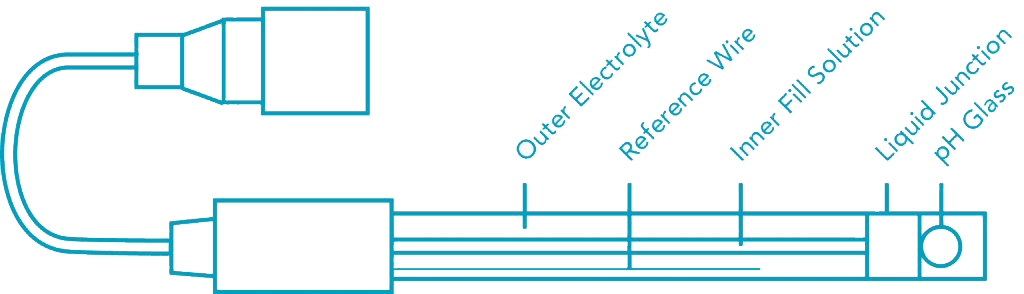
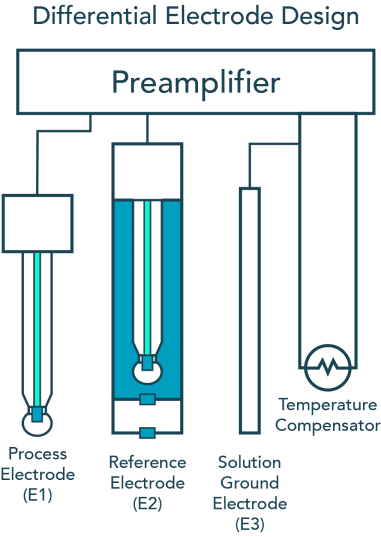
pH, commonly used for water measurements, is a measure of acidity and alkalinity, or the caustic and base present in a given solution. It is generally expressed with a numeric scale ranging from 0-14. The value 7 represents neutrality. The numbers on the scale increase with increasing alkalinity, while the numbers on the scale decrease with increasing acidity.
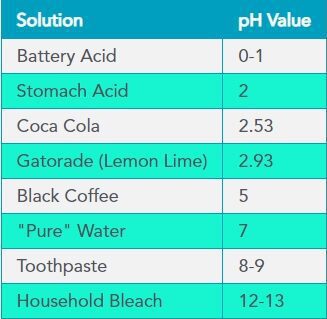
Each unit of change represents a tenfold change in acidity or alkalinity. The pH value is also equal to the negative logarithm of the hydrogen-ion concentration or hydrogen-ion activity. pH values for some common solutions are listed in the table to the right.
Preparing chemical solutions?
Visit our pH calculator first.
How Are pH Measurements Used?
pH measurements are widely used to control processes, ensure product quality and safety.
Custom pH Sensor Design and Manufacturing
In addition to our standard products, Sensorex designs and manufactures pH sensors for over 250 OEM partners. Whether you are looking to private label sensors and instrumentation or incorporate pH sensors as part of the design for your water treatment product, our team of engineers and application specialists can help you meet your goals. We provide custom branding on our standard products and can design custom pH sensors to meet various design specifications.

Popular ProductsSensorex pH Best Sellers
S8300 – Modular pH Sensors for In-line
In heavily contaminated wastewater or chemical processing applications, an inline pH probe typically wears out fast. Constantly replacing an inline pH…
Complete Modular Submersion/Inline* pH Smart Sensors
Sensorex 8000 series Smart Sensors can be configured in countless variations to meet our exact needs. This is one of…
S272CD – Process pH Sensor
The S272CD online process pH sensor delivers reliable online monitoring for in-line or submersion installation configuration. This sensor is designed…
TX2000 – Intelligent pH & ORP Transmitter/Controller
Our TX2000 is an intelligent pH and ORP transmitter/controller for reading pH and ORP sensors and programmable process controls. The large…
S175CD/BNC – pH Sensor, Spear Tip
Looking to measure the pH of substrate, soil, meat, or cheese? A standard pH sensor with bulb shaped glass won't…
SG354/SG355 Glass-Body Flat Surface pH and ORP Electrodes
SG354/SG355 sensors feature a special high molarity reference design for stability, reliability and maximum service life in demanding pool and…
S200CD/BNC – pH Sensor, Laboratory and General Use, Extended Life
Applications such as environmental and wastewater sampling involve some uncertainty about what samples may contain. When dealing with unknown or…
S200CD/10/BNC – Process pH Sensor, Light Duty
The S200CD/10/BNC is a light duty pH probe for continuous monitoring of surface water and ground water, including environmental and…
Shop NowMore from Sensorex
In addition to pH sensors we have a full range of water quality sensors.
HAVE A QUESTION?Get In Touch
If you need help selecting the right sensor for your application drop us a message.