Oxidation Reduction Potential (ORP)
What is ORP?
Oxidation reduction potential (ORP), also known as REDOX, is a measurement that reflects the ability of a molecule to oxidize or reduce another molecule:
- Oxidation is the loss of electrons, so oxidizers accept electrons from other molecules
- Reduction is the gain of electrons, so reducers donate electrons to other molecules
Oxidation reduction potential is measured as a single voltage in millivolts (mV). Oxidizers have a positive ORP value, while reducers have a negative ORP value.
Preparing chemical solutions?
Visit our pH calculator first.
How is ORP Measured?
ORP is measured using an electrochemical sensor called an ORP or REDOX sensor. Similar to to pH sensors, the most common type of ORP sensor is a combination sensor with a measuring electrode and a reference electrode. The measuring cell, typically a noble metal like platinum or gold, detects changes in REDOX potential, while the reference provides a stable comparison signal.
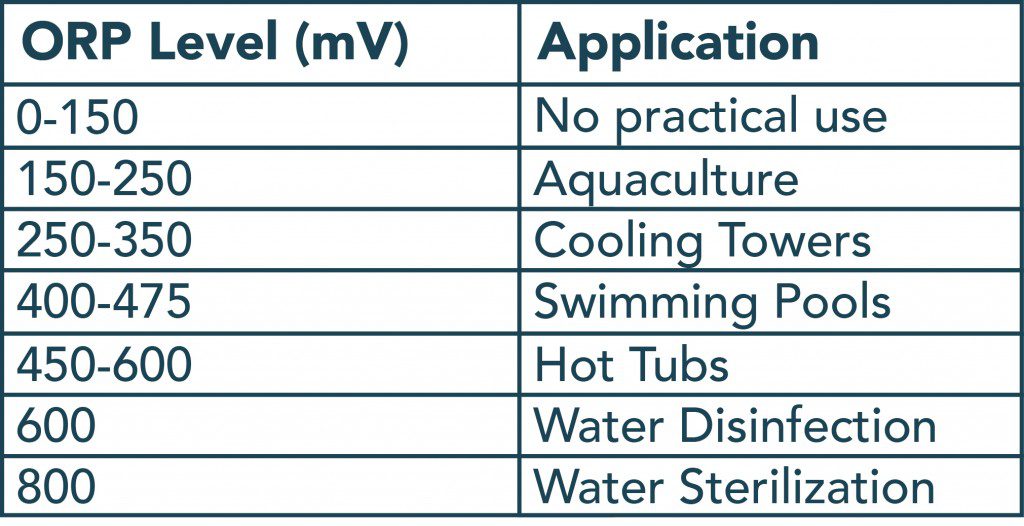
Like a pH measurement, an ORP measurement is not a direct indicator of concentration, but rather an indicator of activity. However, in solutions with one active species, like pool water containing chlorine, ORP correlates to concentration.
Popular ProductsSensorex ORP Best Sellers
S272CD-ORP-MA and S272CD-ORP-MB – Smart ORP Sensors, Online Process
Moderately contaminated water, such as industrial source water or natural water resources, may contain chemicals and debris that damage ORP…
TX2000 – Intelligent pH & ORP Transmitter/Controller
Our TX2000 is an intelligent pH and ORP transmitter/controller for reading pH and ORP sensors and programmable process controls. The large…
Double-junction, Plastic Body Flat pH and ORP Electrodes with DIN 13.5 Cap
Our S354CD electrodes have a rugged flat measurement surface, which breaks less easily when it comes in contact with hard…
Sensorex Smart Sensor User Interface Software
Sensorex Smart Sensor User Interface Software is the perfect partner for your Sensorex Smart Probe or Smart Probe modular system.…
TX105 – pH/ORP Transmitter, Loop Powered 4-20mA
Our TX105 is a fully-featured ORP/pH transmitter for use with pH and ORP sensors. The large display shows pH/ORP value simultaneously…
EM805-EC – Smart Conductivity Modules (4-20mA or MODBUS/RS485) for S8000 Series
The EM805-EC is our most cost-effective solution for connecting our S8000 series Contacting Conductivity sensors to a PLC or SCADA…
EM805 – Smart modules (4-20mA or MODBUS/RS485) for S8000 Series
The EM805 is our most cost-effective solution for connecting our S8000 series pH and ORP sensors to a PLC or…
S8100 – Process ORP Sensor, Submersion
Our S8000 series ORP sensors deliver the most reliable online ORP monitoring in a unique, modular package that minimizes total…
Shop nowMore from Sensorex
In addition to ORP sensors we have a full range of water quality sensors.
HAVE A QUESTION?Get In Touch
If you need help selecting the right sensor for your application drop us a message.